ECU offering new non-drug treatment for depression
GREENVILLE, N.C. (Sept. 6, 2013) — People in eastern North Carolina who are living with depression now have a new, non-invasive, non-drug treatment available.
Psychiatrists at the Brody School of Medicine at East Carolina University are working with a new treatment called repetitive transcranial magnetic stimulation, or rTMS. The treatment is delivered via a device called the NeuroStar TMS Therapy system and is approved by the federal Food and Drug Administration for the treatment of major depressive disorder.

Dr. Sy Saeed
“We are excited to be providing rTMS therapy and to be a part of this major step forward in psychiatry,” said Dr. Sy Saeed, professor and chair of the Department of Psychiatric Medicine at the Brody School of Medicine. “Many patients with this debilitating disease do not benefit from and/or are intolerant of antidepressant medications. Now, we have another treatment option for our patients, who continue to have depressive symptoms despite medication treatments.”
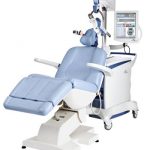
The therapy is an outpatient treatment. It is available by prescription only and delivered under the supervision of a psychiatrist. It is a 37-minute outpatient procedure administered daily for 4-6 weeks.
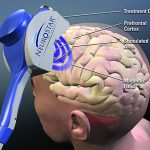
During the therapy session, the patient is awake in a comfortable chair. A small curved device, about the size of a cupped hand, rests on the patient’s head, delivering focused magnetic stimulation directly to the left prefrontal cortex of the brain, an area thought to be involved with regulating mood. The magnetic field pulses of rTMS therapy are the same strength as those used in magnetic resonance imaging machines.
The rTMS therapy is proven safe and effective for treatment-resistant major depressive disorder in adults. More than 10,000 active TMS treatments have been performed with no reported systemic side effects commonly associated with antidepressant medications, according to a 2008 study in the Journal of Clinical Psychiatry. The most common side effect is scalp pain or discomfort, generally mild to moderate.
“Studies demonstrated that treatment with rTMS therapy is safe and effective in patients who did not respond to prior antidepressant therapy,” said Saeed. “In clinical trials, patients suffering with depression experienced a significant improvement in symptoms without side effects that are common with antidepressant medications.”
Depression affects at least 14 million American adults each year, according to a 2005 article in the Archives of General Psychiatry. Other studies have shown that in 2000, the economic burden of depression was estimated at $83.1 billion in the United States, and researchers estimate that by the year 2020, depression will be the second-leading cause of disability worldwide.
Depression can be lethal. Reports show that more than 30,000 people die of suicide each year in the United States, 60 percent of whom suffer from depression.
Overall, women are almost twice as likely as men to suffer from depression. However, some experts feel that depression in men is under-reported. Depression has no racial, ethnic or socioeconomic boundaries.
In a randomized, controlled trial, patients treated with active rTMS therapy experienced an average reduction in their depression symptoms of 22.1 percent compared to a 9 percent average reduction of depression symptoms in patients receiving inactive treatment.
For more information about treatment with rTMS at the ECU Physicians Outpatient Psychiatry Clinic, call 252-744-1406.
- The patient sits in a chair, and a device that emits magnetic waves is positioned on the patient’s head. Contributed image
- The system emits a magnetic field that stimulates areas of the brain thought to be associated with mood. Contributed image