COVID-19 CLINICAL TRIAL
ECU, Vidant begin trial of COVID-19 treatment
A new clinical trial headed by an East Carolina University infectious disease specialist at Vidant Medical Center aims to prevent COVID-19 from causing the type of lung damage in patients that is often fatal.
Dr. Paul Cook, chief of the Division of Infectious Diseases at ECU’s Brody School of Medicine, began enrolling patients June 1 in a clinical trial investigating a new treatment aimed at preventing and shortening the duration of Acute Respiratory Distress Syndrome (ARDS). ARDS is a characteristic of COVID-19 that occurs when fluid builds up in the lungs and prevents oxygen from entering the bloodstream.
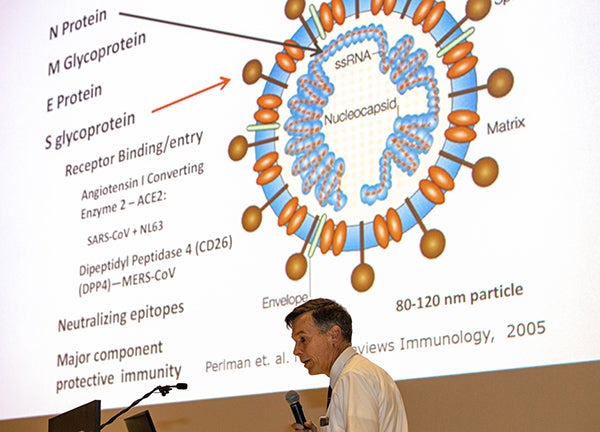
Dr. Paul Cook, chief of the Division of Infectious Disease at the Brody School of Medicine, gives a talk about coronavirus at ECU’s Black Box Theater on March 2, 2020. Cook is heading a clinical trial of a drug aimed at preventing COVID-19 from causing the type of lung damage in patients that is often fatal. (Photo by Cliff Hollis)
ECU is one of only eight sites in the United States participating in the trial, which is a collaboration with pharmaceutical company Eli Lilly and Vidant Medical Center.
The drug, which does not yet have a name but is referred to as LY3127804, is a monoclonal antibody to Angiopoietin-2 (Ang2). Ang2 acts on endothelial cells in the lungs, causing them to become leaky. Ang2 levels are known to be elevated in ARDS patients. The trial will test whether preventing the effects of Ang2 with this monoclonal antibody can reduce the progression to ARDS or the need for ventilators in COVID-19 patients.
Prior to COVID-19, Eli Lilly was developing the drug to treat ARDS associated with cancer and cerebral malaria, a condition most prevalent in Africa.
“Basically, by getting this one-time infusion, this monoclonal antibody binds to this substance Angiopoeitin-2 and prevents it from causing the damage in the lung that is responsible for the Acute Respiratory Distress Syndrome,” Cook said, adding that most of the deaths attributed to COVID-19 are related to complications from ARDS. “We suspect that this drug will reduce development of ARDS, reduce need for mechanical ventilation, reduce hospital days and reduce mortality related to Covid-19.”
Monoclonal antibodies are typically low risk in terms of side effects that patients experience, Cook said. This, coupled with an understanding of how Ang2 can cause ARDS, gave Cook confidence in the drug’s safety and potential for helping COVID-19 patients.
“A certain percentage — maybe 15-20 percent of people (with COVID-19) are going to get more than just minor symptoms and they get this cytokine storm that leads to the pulmonary problems,” Cook said. “Those people end up in the hospital. They end up — a lot of them — on ventilators, most of them on oxygen, and some of them die. That’s what we’re trying to prevent. This drug, by acting on this Angiopoeitin-2, particularly if it’s given early enough, is likely to reduce the morbidity and severity of the disease.”
The treatment protocol has been approved by the FDA for further study. Cook said he hopes to enroll around 30 patients within a four- to six-week period for the double-blind placebo-controlled trial. Enrollment in the trial is voluntary and free of charge for patients who must exhibit moderate to severe symptoms of COVID-19 in order to be considered.